Physico–chemical treatment techniques for wastewater laden with heavy metals

Physico–chemical treatment techniques for wastewater laden with heavy metals
Tonni Agustiono Kurniawan a, Gilbert Y.S. Chan a,∗, Wai-Hung Lo a,∗, Sandhya Babel b
Introduction
Due to the discharge of large amount of metal-contaminated wastewater, electroplating industry is one of the most hazardous among the chemical-intensive industries [1]. Inorganic effluent from the industries contains toxic metals such as Cd, Cr, Cu, Ni and Zn [2], which tend to accumulate in the food chain.
Because of their high solubility in the aquatic environments, heavy metals can be absorbed by living organisms. Once they enter the food chain, large concentrations of heavy metals may accumulate in the human body. If the metals are ingested beyond the permitted concentration, they can cause serious health disorders (Table 1). Therefore, it is necessary to treat metal-contaminated wastewater prior to its discharge to the environment.
Different treatment techniques for wastewater laden with heavy metals have been developed in recent years both to decrease the amount of wastewater produced and to improve the quality of the treated effluent. Although various treatments such as chemical precipitation, coagulation–flocculation, flotation, ion exchange and membrane filtration can be employed to remove heavy metals from contaminated wastewater, they have their inherent advantages and limitations in application.
Chemical precipitation is widely used for the treatment of electroplating wastewater in Thailand [7] and Turkey [8]. Coagulation–flocculation has also been employed for heavy metal removal from inorganic effluent in Thailand [7] and China [9]. Sorptive flotation has attracted interest in Greece [10] and the USA [11] for the removal of non-surface-active metal ions from contaminated wastewater. In recent years, ion exchange has also received considerable interest in Italy [12] and Spain [13] as one of the most promising methods to treat wastewaters laden with heavy metals.
Due to its convenient operation, membrane separation has been increasingly used recently for the treatment of inorganic effluent. There are different types of membrane filtration such as ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO). Membrane filtration has been used in Taiwan [14] and South Korea [15] to remove Cd(II), Ni(II), Zn(II) and Cr(III) ions from contaminated wastewater.
Electro treatments such as electrodialysis [16], membrane electrolysis [17] and electrochemical precipitation [18] have also contributed to environmental protection. However, these techniques have been investigated less extensively due to the high operational cost caused by energy consumption. Although many techniques can be employed for the treatment of inorganic effluent, the ideal treatment should be not only suitable, appropriate and applicable to the local conditions, but also able to meet the maximum contaminant level (MCL) standards established (Table 1) [19].
This article presents an overview with critical analysis of the technical applicability of various physico–chemical treatments for wastewater laden with heavy metals. Their advantages and limitations in application are evaluated. To highlight their removal performance, the operating conditions such as pH, dose required, initial metal concentration and treatment efficiency are presented as well.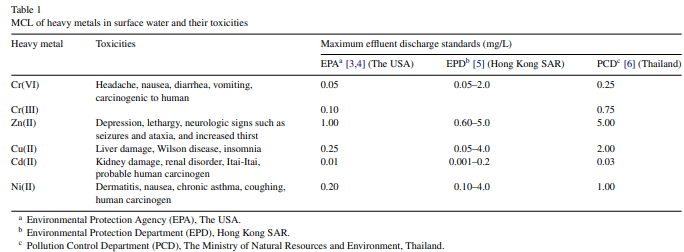
2. Physico–chemical treatment techniques for wastewater laden with heavy metals
2.1. Chemical precipitation
Chemical precipitation is widely used for heavy metal removal from inorganic effluent [20,21]. After pH adjustment to the basic conditions (pH 11), the dissolved metal ions are converted to the insoluble solid phase via a chemical reaction with a precipitant agent such as lime [22]. Typically, the metal precipitated from the solution is in the form of hydroxide [23]. The conceptual mechanism of heavy metal removal by chemical precipitation is presented in Eq. (1) [22]:
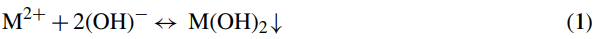
where M2+ and OH− represent the dissolved metal ions and the precipitant, respectively, while M(OH)2 is the insoluble metal hydroxide.
Lime precipitation was employed for the removal of heavy metals such as Zn(II), Cd(II) and Mn(II) cations with initial metal concentrations of 450, 150 and 1085 mg/L, respectively, in a batch continuous system [7]. In spite of their varying initial concentrations, an almost complete removal from synthetic wastewater was achieved for all the metals at pH 11, complying with the effluent limit of the Thai Pollution Control Department for Zn(II) and Mn(II) of less than 5 mg/L [6]. However, the treated effluent was unable to meet the stringent limit set by the US EPA of lower than 1 mg/L [3,4], thus suggesting that subsequent treatments using other physico–chemical methods were still required to comply with the US EPA discharge standard.
Some attractive findings were reported by Tunay and Kabdasli[8], who investigated the applicability of hydroxide precipitation in a closed system to treat synthetic wastewater containing Cd(II) and Cu(II) ions. Inorganic cations (Ca(II) and Na) were employed as ligand-sharing agents for EDTA (ethylenediaminetetraacetic acid) and NTA (nitrilotriacetic acid). They reported that Ca(II) was the only cation that effectively bound both ligands to form the hydroxide precipitations of the complexed metals. At pH 11, EDTA was also found to be the major component that determined Cd(II) solubility [24]. The removal performance of the precipitants is presented in Table 2.
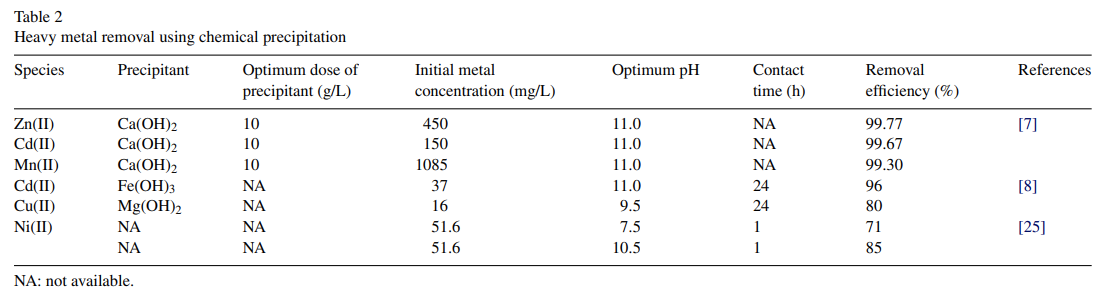
Different results were obtained for the removal of Ni(II) uptake from a low-strength of real wastewater with a metal concentration of less than 100 mg/L [25]. At pH 7.5 and 10.5, the researchers found that about 71% and 85% of Ni(II) removal, respectively, with an initial metal concentration of 51.6 mg/L, could be attained. This could be attributed to the fact that a greater portion of the Ni(II) was precipitated and removed in the form of insoluble hydroxide compounds with the increasing pH.
Overall, pH adjustment to the basic conditions (pH 11) is the major parameter that significantly improves heavy metal removal by chemical precipitation (Table 2). Due to its availability in most countries, lime or calcium hydroxide is the most commonly employed precipitant agent. Lime precipitation can be employed to effectively treat inorganic effluent with a metal concentration of higher than 1000 mg/L. Other advantages of using lime precipitation include the simplicity of the process, inexpensive equipment requirement, and convenient and safe operations, making it a popular method for metal removal from contaminated wastewater.
In spite of its advantages, chemical precipitation requires a large amount of chemicals to reduce metals to an acceptable level for discharge [26]. Other drawbacks are its excessive sludge production that requires further treatment, the increasing cost of sludge disposal, slow metal precipitation, poor settling, the aggregation of metal precipitates, and the long-term environmental impacts of sludge disposal [27–29].
2.2. Coagulation–flocculation
Coagulation–flocculation can be employed to treat wastewater laden with heavy metals. Principally, the coagulation process destabilizes colloidal particles by adding a coagulant and results in sedimentation [30]. To increase the particle size, coagulation is followed by the flocculation of the unstable particles into bulky floccules [31]. The general approach for this technique includes pH adjustment and involves the addition of ferric/alum salts as the coagulant to overcome the repulsive forces between particles [32].
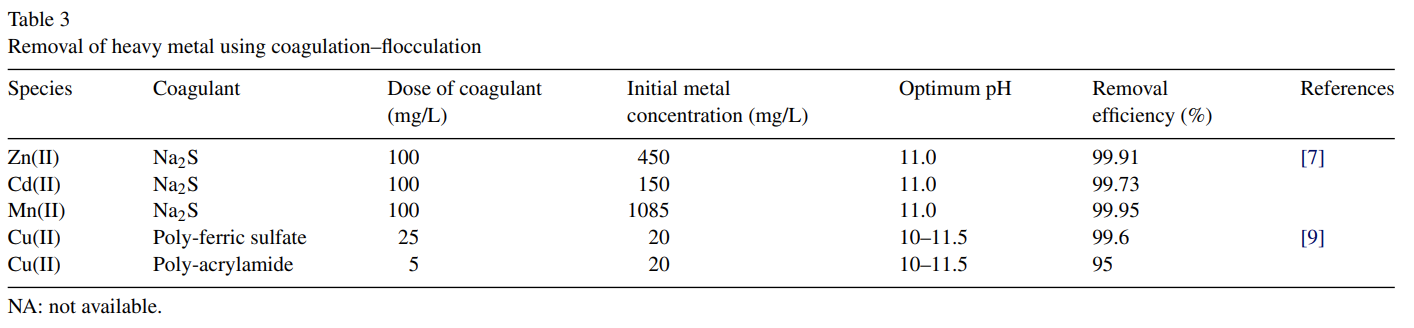
After lime precipitation, Charerntanyarak [7] employed subsequent coagulation process to remove Zn(II), Cd(II) and Mn(II) ions from synthetic wastewater. The optimum pH for coagulation process was found to be 11. At pH 11, the concentration of Zn(II) and Mn(II) in the treated effluent was reduced to less than 5 mg/L, the limit for the wastewater discharge set by the Thai Pollution Control Department [6].
To treat real electroplating wastewater containing copper, Li et al. [9] modified the conventional coagulation–flocculation process by using sodium diethyl-dithiocarbamate (DDTC) as a trapping agent and both poly-ferric sulphate and polyacrylamide as the flocculants. DDTC is the most common chemical used as metal precipitant to form insoluble metal-dithio salts [33]. These insoluble dithio-metal salts are then coprecipitated, forming hydroxide-neutralized solids that precipitate prior to the discharge of the treated waste stream. When the mole ratio of DDTC to Cu was between 0.8 and 1.2, they found that an almost complete removal of Cu(II) could be achieved (Table 3).
In general, coagulation–flocculation can treat inorganic effluent with a metal concentration of less than 100 mg/L or higher than 1000 mg/L. Like chemical precipitation, pH ranging from 11.0 to 11.5 has been found to be effective to improve the heavy metal removal by the coagulation–flocculation process (Table 3). Improved sludge settling, dewatering characteristics, bacterial inactivation capability, sludge stability are reported to be the major advantages of lime-based coagulation [34,35].
In spite of its advantages, coagulation–flocculation has limitations such as high operational cost due to chemical consumption. The increased volume of sludge generated from coagulation–flocculation may hinder its adoption as a global strategy for wastewater treatment. This can be attributed to the fact that the toxic sludge must be converted into a stabilized product to prevent heavy metals from leaking into the environment [36].
To overcome such problems, electro-coagulation may be a better alternative than conventional coagulation, as it can remove the smallest colloidal particles and produce just a small amount of sludge [37,38]. However, this technique also creates a floc of metallic hydroxides, which requires further purification [39], making the recovery of valuable heavy metals impossible.
2.3. Flotation
Flotation is employed to separate solids or dispersed liquids from a liquid phase using bubble attachment [40]. The attached particles are separated from the suspension of heavy metal by the bubble rise. Flotation can be classified as: (i) dispersed-air flotation, (ii) dissolved-air flotation (DAF), (iii) vacuum air flotation, (iv) electro flotation, and (v) biological flotation. Among the various types of flotation, DAF is the most commonly used for the treatment of metal-contaminated wastewater [41]. Adsorptive bubble separation employs foaming to separate the metal impurities. The target floating substances are separated from bulk water in a foaming phase.
Laboratory study was carried out by Rubio and Tessele [42] to investigate the flotation of Zn(II) and Ni(II) from synthetic wastewater using chabazite as the adsorptive particulate. They found that the removal performance was dependent on the interfacial chemistry and aggregation effectiveness. An almost complete removal (98.6%) of heavy metal ions with an initial concentration of 2 mg/L could be achieved by using 20 mg/L of Fe(OH)3. The results are comparable to those of Blocher et ¨ al. [43], who combined flotation and membrane separations to remove Ni(II) cations from synthetic plating solution by using CTABr (cetyl trimethyl-ammonium bromide) as the cationic collector (Table 4).
Other interesting results are reported by Zamboulis et al. [44], who studied the sorptive flotation for the removal of Zn(II) and Cu(II) ions from synthetic wastewater. SDS (sodium dodecyl sulfate) and HDTMA (hexadecyl-trimethyl-ammoniumbromide) were used as the cationic collectors. The addition of 2 g/L of zeolite was found to remove 99% of 50 mg/L of zinc(II), while 4 g/L of zeolite was required to remove 97% of 500 mg/L of the initial Cu(II) concentration (Table 4). The results confirmed that both the surface charge of the system and the solution pH significantly affected the metal removal by zeolite [10].
To explore their application as biosurfactants, Surfactin105 and Lychenysin-A were applied to enhance the removal of Cr(VI) and Zn(II) ions from synthetic wastewater [45]. An almost complete removal of both metals with initial metal concentrations of 50 mg/L could be achieved at pH 4.0.
In the last decade, the trends of research had shifted from flotation alone to a combination of flotation and other physico–chemical treatments such as filtration or powder activated carbon [46,47]. Encouraging results were reported by Mavrov et al. [48], who examined a newly integrated process that combined adsorption, membrane separation and flotation for Cu(II) and Zn(II) removal from real wastewater with synthetic zeolite as the bonding agent. They found that about 97% of Cu(II) and Zn(II) removal were attained with an initial metal concentration of 60 mg/L. The binding capacities of Zn(II), Cu(II) and Ni(II) ions were found to be 270, 200 and 60 mg/g, respectively. The results were higher than those of Doyle and Liu [11], who employed triethylenetetraamine (Trien) as the collector for the flotation of Cu(II) ions from synthetic wastewater (Table 4).
Although it is only a kind of physical separation process, heavy metal removal by flotation has the potential for industrial application [49]. Low-cost materials such as zeolite and chabazite have been found to be effective collectors with removal efficiency of higher than 95% for an initial metal concentration ranging from 60 to 500 mg/L (Table 4). Flotation can be employed to treat inorganic effluent with a metal concentration of less than 50 mg/L or higher than 150 mg/L. Other advantages such as better removal of small particles, shorter hydraulic retention times and low cost make flotation one of the most promising alternatives for the treatment of metal-contaminated wastewater [50,51].
2.4. Membrane filtration
Membrane filtration has received considerable attention for the treatment of inorganic effluent, since it is capable of removing not only suspended solid and organic compounds, but also inorganic contaminants such as heavy metals. Depending on the size of the particle that can be retained, various types of membrane filtration such as ultrafiltration, nanofiltration and reverse osmosis can be employed for heavy metal removal and are presented as follows.
2.4.1. Ultrafiltration (UF)
UF utilizes permeable membrane to separate heavy metals, macromolecules and suspended solids from inorganic solution on the basis of the pore size (5–20 nm) and molecular weight of the separating compounds (1000–100,000 Da) [52]. These unique specialties enable UF to allow the passage of water and low-molecular weight solutes, while retaining the macromolecules, which have a size larger than the pore size of the membrane [53].
Some significant findings were reported by Juang and Shiau [14], who studied the removal of Cu(II) and Zn(II) ions from synthetic wastewater using chitosan-enhanced membrane filtration. The amicon-generated cellulose YM10 was used as the ultrafilter. About 100% and 95% rejection were achieved at pH ranging from 8.5 to 9.5 for Cu(II) and Zn(II) ions, respectively, with an initial Cu(II) concentration of 79 mg/L and Zn(II) concentration of 81 mg/L. The results indicated that chitosan significantly enhanced metals removal by 6–10 times compared to using membrane alone. This could be attributed to the major role of the amino groups of the chitosan chain, which served as coordination sites for metal binding. In acidic conditions, the amino groups of chitosan are protonated after reacting with H+ ions as follows:
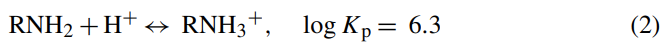
Having the unshared electron pair of the nitrogen atom as the sole electron donor, the non-protonated chitosan binds with the unsaturated transition metal cation through the formation of coordination bond [54]. For most of the chelating adsorbent, the functional groups with the donor atoms are normally attached to the metal ions, thus leading to a donor–acceptor interaction between chitosan and the metal ions [55], as indicated by the
Eq. (3):
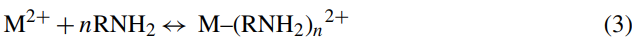
where M and RNH2 represent metal and the amino group of chitosan, respectively, while n is the number of the unprotonated chitosan bound to the metal. Combination of Eqs. (2) and (3) gives the overall reaction as follows:
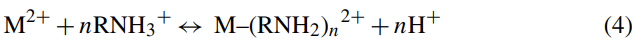
Eq. (4) suggests that an increase in pH would enhance the formation of metal–chitosan complexes.
To improve the rejection rates of metals by complexation– ultrafiltration, some modifications were conducted using polyethylene-imine (PEI), a water-soluble macroligand, to remove Cr(III) ions from a synthetic solution [56]. Technical parameters such as pH, ligand concentration, applied pressure and membrane pore size were found to significantly affect the rejection rate of metal ions. The researchers found that pH 6.0, pressure of 3 bar and 2 g/L of PEI were the optimum conditions to achieve the Cr rejection rate of 95% with an initial metal concentration of 20 mg/L (Table 5).
Another significant breakthrough in UF research was explored for the removal of Ni(II) ions from a synthetic solution using micellar-enhanced UF [57]. Both sodium dodecyl sulfate (SDS) and non-ionic mono-alkylphenol polyetoxilate were used to form micelles. An almost complete removal of Ni(II) could be achieved at 4 bar of pressure with 1 g/L of SDS concentration. This result was higher than that of Akita et al. [58], who studied the removal of Ni(II) ions from synthetic wastewater via micellar-enhanced UF. They found that only 60% of Ni(II) with an initial metal concentration of 29 mg/L was removed at pH ranging from 5 to 7, confirming that the metal rejection rates were dependent on the degree of complexation between the cations and the extractant within the micelle [59].
To explore its potential to remove heavy metals, Saffaj et al. [60] employed low cost ZnAl2O4–TiO2 UF membranes for the removal of Cd(II) and Cr(III) ions from synthetic solution. They reported that 93% Cd(II) rejection and 86% Cr(III) rejection were achieved (Table 5). Such high rejection rates might be attributed to the strong interactions between the divalent cations and the positive charge of the membranes. These results indicate that the charge capacity of the UF membrane, the charge valencies of the ions and the ion concentration in the effluent, played major roles in determining the ion rejection rates by the UF membranes [61].
Depending on the membrane characteristics, UF can achieve more than 90% of removal efficiency with a metal concentration ranging from 10 to 112 mg/L at pH ranging from 5 to 9.5 and at 2–5 bar of pressure (Table 5). UF presents some advantages such as a lower driving force and a smaller space requirement due to its high packing density. However, the decrease in UF performance due to membrane fouling has hindered it from a wider application in wastewater treatment. Fouling has many adverse effects on the membrane system such as flux decline, an increase in transmembrane pressure (TMP) and the biodegradation of the membrane materials [62]. These effects result in high operational costs for the membrane system.
References
[1] S. Babel, T.A. Kurniawan, Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan, Chemosphere 54 (7) (2004) 951–967.
[2] T.A. Kurniawan, A research study on Cr(VI) removal from electroplating wastewater using chemically modified low-cost adsorbents and commercial activated carbon, Sirindhorn International Institute of Technology (SIIT), Thammasat University, Pathumthani, ISBN 974- 570-828-3 (Master thesis), 2002.
[3] US Environmental Protection Agency (EPA), Development Document for Effluent Limitations Guidelines and Standards for the Metal Finishing Point Source Category, US EPA, Washington, DC, 1980 (EPA440/1-80/091-a).
[4] US Environmental Protection Agency (EPA), Control and treatment technology for the metal finishing industry, sulfide precipitation, Summary Report, US EPA, Washington, DC, (EPA-625/8-80/003), 1980.
[5] Environmental Protection Department (EPD), Hong Kong, Technical memorandum standards for effluents discharged into drainage and sewerage systems, inland and coastal water, 2005 [].
[6] Pollution Control Department (PCD), Thai Ministry of Natural Resources and Environment, Water quality standards, 2005 [].
[7] L. Charerntanyarak, Heavy metals removal by chemical coagulation and precipitation, Water Sci. Technol. 39 (10/11) (1999) 135–138.
[8] O. Tunay, N.I. Kabdasli, Hydroxide precipitation of complexed metals, ¨ Water Res. 28 (10) (1994) 2117–2124.
[9] Y.J. Li, X.P. Zeng, Y.F. Liu, S.S. Yan, Z.H. Hu, Ya-Ming, Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation, Sep. Purif. Technol. 31 (2003) 91–95.
[10] N.K. Lazaridis, K.A. Matis, M. Webb, Flotation of metal-loaded clay anion exchangers. Part I: the case of chromate, Chemosphere 42 (2001) 373–378.
[11] F.M. Doyle, Z.D. Liu, The effect of triethylenetetraamine (trien) on the ion flotation of Cu2+ and Ni2+, J. Coll. Int. Sci. 258 (2003) 396–403.
[12] M. Pansini, C. Colella, M. De’Gennaro, Chromium removal from water by ion exchange using zeolite, Desalination 83 (1991) 145–157.
[13] E. Alvarez-Ayuso, A. Garc ´ ´谋a-Sanchez, X. Querol, Purification of metal ´ electroplating wastewaters using zeolites, Water Res. 37 (20) (2003) 4855–4862.
[14] R.S. Juang, R.C. Shiau, Metal removal from aqueous solutions using chitosan-enhanced membrane filtration, J. Membr. Sci. 165 (2000) 159–167.
[15] K.H. Ahn, K.G. Song, H.Y. Cha, I.T. Yeom, Removal of ions in nickel electroplating rinse water using low-pressure nanofiltration, Desalination 122 (1999) 77–84.
[16] A.J. Pedersen, Characterization and electrolytic treatment of wood combustion fly ash for the removal of cadmium, Biomass Bioenergy 25 (4) (2003) 447–458.
[17] L.J.J. Janssen, L. Koene, The role of electrochemistry and electrochemical technology in environmental protection, Chem. Eng. J. 85 (2002) 137–146.
[18] N. Kongsricharoern, C. Polprasert, Electrochemical precipitation of chromium (Cr6+) from an electroplating wastewater, Water Sci. Technol. 31 (9) (1995) 109–117.
[19] S. Babel, T.A. Kurniawan, Various treatment technologies to remove arsenic and mercury from contaminated groundwater: an overview, in: Proceedings of the 1st International Symposium on Southeast Asian Water Environment, Bangkok, Thailand, 24–25 October, 2003, pp. 433–440.
[20] L.D. Benefield, J.M. Morgan, Chemical precipitation, in: R.D. Letterman (Ed.), Water Quality and Treatment, McGraw-Hill Inc., NY, 1999, pp. 10.1–10.57.
[21] US Environmental Protection Agency (EPA), Chemical Precipitation, US EPA, Washington, DC, 2000 (EPA832-F-00-018).
[22] L.K. Wang, D.A. Vaccari, Y. Li, N.K. Shammas, Chemical precipitation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 141–198.
[23] O. Tunay, Developments in the application of chemical technologies ¨ to wastewater treatment, Water Sci. Technol. 48 (11/12) (2003) 43–52.
[24] O. Tunay, N.I. Kabdasli, R. Tasli, Pretreatment of complexed metal ¨ wastewaters, Water Sci. Technol. 29 (9) (1994) 265–274.
[25] A. Papadopoulos, D. Fatta, K. Parperis, A. Mentzis, K.J. Harambous, M. Loizidou, Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods, Sep. Purif. Technol. 39 (3) (2004) 181–188.
[26] K. Juttner, U. Galla, H. Schmieder, Electrochemical approaches to ¨ environmental problems in the process industry, Electrochim. Acta 45 (2000) 2575–2594.
[27] X.J. Yang, A.G. Fane, S. MacNaughton, Removal and recovery of heavy metals from wastewater by supported liquid membranes, Water Sci. Technol. 43 (2) (2001) 341–348.
[28] P. Bose, M.A. Bose, S. Kumar, Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc, and cyanide, Adv. Environ. Res. 7 (2002) 179–195.
[29] U. Wingenfelder, C. Hansen, G. Furrer, R. Schulin, Removal of heavy metals from mine water by natural zeolites, Environ. Sci. Technol. 39 (2005) 4606–4613.
[30] N.K. Shammas, Coagulation and flocculation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 103–140.
[31] L. Semerjian, G.M. Ayoub, High-pH-magnesium coagulation–flocculation in wastewater treatment, Adv. Environ. Res. 7 (2003) 389– 403.
[32] I. Licsko, Realistic coagulation mechanisms in the use of aluminium ´ and iron(III) salts, Water Sci. Technol. 36 (4) (1997) 103–110.
[33] M.E. Andrus, A review of metal precipitation chemicals for metalfinishing applications, Met. Finish. 98 (11) (2000) 20–23.
[34] R.C. Cheng, S. Liang, H.C. Wang, M.D. Beuhler, Enhanced coagulation for arsenic removal, J. AWWA 86 (9) (1994) 79–90.
[35] M. Edwards, Chemistry of arsenic removal during coagulation and Fe–Mn oxidation, J. AWWA 86 (9) (1994) 64–78.
[36] G.M. Ayoub, L. Semerjian, A. Acra, M. El Fadel, B. Koopman, Heavy metal removal by coagulation with seawater liquid bittern, J. Environ. Eng. 127 (3) (2001) 196–202.
[37] E.I. Vik, D.A. Carlsoon, A.S. Eikum, E.T. Gjessing, Electrocoagulation of potable water, Water Res. 18 (1984) 1355–1360.
[38] M. Elimelech, C.R. O’Melia, Kinetics of deposition of colloidal particles in porous media, Environ. Sci. Technol. 24 (1990) 1528–1536.
[39] F. Persin, M. Rumeau, Le traitement electrochimique des eaux et des ´ effluents, Tribune de l’eau 3 (42) (1989) 45–46. [40] L.K. Wang, E.M. Fahey, Z.C. Wu, Dissolved air flotation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 431–500.
[41] T. Zabel, Flotation in water treatment, in: K.J. Ives (Ed.), The Scientific Basis of Flotation, Martinus Nijhoff Publishers, The Hague, 1984, pp. 349–378.
[42] J. Rubio, F. Tessele, Removal of heavy metal ions by adsorptive particulate flotation, Min. Eng. 10 (7) (1997) 671–679.
[43] C. Blocher, J. Dorda, V. Mavrov, H. Chmiel, N.K. Lazaridis, K.A. ¨ Matis, Hybrid flotation-membrane filtration processes for the removal of heavy metal ions from wastewater, Water Res. 37 (2003) 4018–4026.
[44] D. Zamboulis, S.I. Pataroudi, A.I. Zouboulis, K.A. Matis, The application of sorptive flotation for the removal of metal ions, Desalination 162 (2004) 159–168.
[45] A.I. Zouboulis, K.A. Matis, N.K. Lazaridis, P.N. Golyshin, The use of biosurfactants in flotation: application for the removal of metal ions, Min. Eng. 16 (2003) 1231–1236.
[46] G. Offringa, Dissolved air flotation in Southern Africa, Water Sci. Technol. 31 (3/4) (1995) 159–172.
[47] S. Laine, T. Poujol, S. Dufay, J. Baron, P. Robert, Treatment of ´ stormwater to bathing water quality by dissolved air flotation, filtration and ultraviolet disinfection, Water Sci. Technol. 38 (10) (1998) 99–105.
[48] V. Mavrov, T. Erwe, C. Blocher, H. Chmiel, Study of new integrated ¨ processes combining adsorption, membrane separation and flotation for heavy metal removal from wastewater, Desalination 157 (2003) 97–104.
[49] P. Jokela, P. Keskitalo, Plywood mill water system closure by dissolved air flotation treatment, Water Sci. Technol. 40 (11/12) (1999) 33–42.
[50] K.A. Matis, A.I. Zouboulis, N.K. Lazaridis, I.C. Hancock, Sorptive flotation for metal ions recovery, Int. J. Miner. Process 70 (2003) 99–108.
Tonni Agustiono Kurniawan a, Gilbert Y.S. Chan a,∗, Wai-Hung Lo a,∗, Sandhya Babel b
Introduction
Due to the discharge of large amount of metal-contaminated wastewater, electroplating industry is one of the most hazardous among the chemical-intensive industries [1]. Inorganic effluent from the industries contains toxic metals such as Cd, Cr, Cu, Ni and Zn [2], which tend to accumulate in the food chain.
Because of their high solubility in the aquatic environments, heavy metals can be absorbed by living organisms. Once they enter the food chain, large concentrations of heavy metals may accumulate in the human body. If the metals are ingested beyond the permitted concentration, they can cause serious health disorders (Table 1). Therefore, it is necessary to treat metal-contaminated wastewater prior to its discharge to the environment.
Different treatment techniques for wastewater laden with heavy metals have been developed in recent years both to decrease the amount of wastewater produced and to improve the quality of the treated effluent. Although various treatments such as chemical precipitation, coagulation–flocculation, flotation, ion exchange and membrane filtration can be employed to remove heavy metals from contaminated wastewater, they have their inherent advantages and limitations in application.
Chemical precipitation is widely used for the treatment of electroplating wastewater in Thailand [7] and Turkey [8]. Coagulation–flocculation has also been employed for heavy metal removal from inorganic effluent in Thailand [7] and China [9]. Sorptive flotation has attracted interest in Greece [10] and the USA [11] for the removal of non-surface-active metal ions from contaminated wastewater. In recent years, ion exchange has also received considerable interest in Italy [12] and Spain [13] as one of the most promising methods to treat wastewaters laden with heavy metals.
Due to its convenient operation, membrane separation has been increasingly used recently for the treatment of inorganic effluent. There are different types of membrane filtration such as ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO). Membrane filtration has been used in Taiwan [14] and South Korea [15] to remove Cd(II), Ni(II), Zn(II) and Cr(III) ions from contaminated wastewater.
Electro treatments such as electrodialysis [16], membrane electrolysis [17] and electrochemical precipitation [18] have also contributed to environmental protection. However, these techniques have been investigated less extensively due to the high operational cost caused by energy consumption. Although many techniques can be employed for the treatment of inorganic effluent, the ideal treatment should be not only suitable, appropriate and applicable to the local conditions, but also able to meet the maximum contaminant level (MCL) standards established (Table 1) [19].
This article presents an overview with critical analysis of the technical applicability of various physico–chemical treatments for wastewater laden with heavy metals. Their advantages and limitations in application are evaluated. To highlight their removal performance, the operating conditions such as pH, dose required, initial metal concentration and treatment efficiency are presented as well.

2. Physico–chemical treatment techniques for wastewater laden with heavy metals
2.1. Chemical precipitation
Chemical precipitation is widely used for heavy metal removal from inorganic effluent [20,21]. After pH adjustment to the basic conditions (pH 11), the dissolved metal ions are converted to the insoluble solid phase via a chemical reaction with a precipitant agent such as lime [22]. Typically, the metal precipitated from the solution is in the form of hydroxide [23]. The conceptual mechanism of heavy metal removal by chemical precipitation is presented in Eq. (1) [22]:
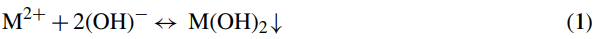
where M2+ and OH− represent the dissolved metal ions and the precipitant, respectively, while M(OH)2 is the insoluble metal hydroxide.
Lime precipitation was employed for the removal of heavy metals such as Zn(II), Cd(II) and Mn(II) cations with initial metal concentrations of 450, 150 and 1085 mg/L, respectively, in a batch continuous system [7]. In spite of their varying initial concentrations, an almost complete removal from synthetic wastewater was achieved for all the metals at pH 11, complying with the effluent limit of the Thai Pollution Control Department for Zn(II) and Mn(II) of less than 5 mg/L [6]. However, the treated effluent was unable to meet the stringent limit set by the US EPA of lower than 1 mg/L [3,4], thus suggesting that subsequent treatments using other physico–chemical methods were still required to comply with the US EPA discharge standard.
Some attractive findings were reported by Tunay and Kabdasli[8], who investigated the applicability of hydroxide precipitation in a closed system to treat synthetic wastewater containing Cd(II) and Cu(II) ions. Inorganic cations (Ca(II) and Na) were employed as ligand-sharing agents for EDTA (ethylenediaminetetraacetic acid) and NTA (nitrilotriacetic acid). They reported that Ca(II) was the only cation that effectively bound both ligands to form the hydroxide precipitations of the complexed metals. At pH 11, EDTA was also found to be the major component that determined Cd(II) solubility [24]. The removal performance of the precipitants is presented in Table 2.

Different results were obtained for the removal of Ni(II) uptake from a low-strength of real wastewater with a metal concentration of less than 100 mg/L [25]. At pH 7.5 and 10.5, the researchers found that about 71% and 85% of Ni(II) removal, respectively, with an initial metal concentration of 51.6 mg/L, could be attained. This could be attributed to the fact that a greater portion of the Ni(II) was precipitated and removed in the form of insoluble hydroxide compounds with the increasing pH.
Overall, pH adjustment to the basic conditions (pH 11) is the major parameter that significantly improves heavy metal removal by chemical precipitation (Table 2). Due to its availability in most countries, lime or calcium hydroxide is the most commonly employed precipitant agent. Lime precipitation can be employed to effectively treat inorganic effluent with a metal concentration of higher than 1000 mg/L. Other advantages of using lime precipitation include the simplicity of the process, inexpensive equipment requirement, and convenient and safe operations, making it a popular method for metal removal from contaminated wastewater.
In spite of its advantages, chemical precipitation requires a large amount of chemicals to reduce metals to an acceptable level for discharge [26]. Other drawbacks are its excessive sludge production that requires further treatment, the increasing cost of sludge disposal, slow metal precipitation, poor settling, the aggregation of metal precipitates, and the long-term environmental impacts of sludge disposal [27–29].
2.2. Coagulation–flocculation
Coagulation–flocculation can be employed to treat wastewater laden with heavy metals. Principally, the coagulation process destabilizes colloidal particles by adding a coagulant and results in sedimentation [30]. To increase the particle size, coagulation is followed by the flocculation of the unstable particles into bulky floccules [31]. The general approach for this technique includes pH adjustment and involves the addition of ferric/alum salts as the coagulant to overcome the repulsive forces between particles [32].
After lime precipitation, Charerntanyarak [7] employed subsequent coagulation process to remove Zn(II), Cd(II) and Mn(II) ions from synthetic wastewater. The optimum pH for coagulation process was found to be 11. At pH 11, the concentration of Zn(II) and Mn(II) in the treated effluent was reduced to less than 5 mg/L, the limit for the wastewater discharge set by the Thai Pollution Control Department [6].
To treat real electroplating wastewater containing copper, Li et al. [9] modified the conventional coagulation–flocculation process by using sodium diethyl-dithiocarbamate (DDTC) as a trapping agent and both poly-ferric sulphate and polyacrylamide as the flocculants. DDTC is the most common chemical used as metal precipitant to form insoluble metal-dithio salts [33]. These insoluble dithio-metal salts are then coprecipitated, forming hydroxide-neutralized solids that precipitate prior to the discharge of the treated waste stream. When the mole ratio of DDTC to Cu was between 0.8 and 1.2, they found that an almost complete removal of Cu(II) could be achieved (Table 3).
In general, coagulation–flocculation can treat inorganic effluent with a metal concentration of less than 100 mg/L or higher than 1000 mg/L. Like chemical precipitation, pH ranging from 11.0 to 11.5 has been found to be effective to improve the heavy metal removal by the coagulation–flocculation process (Table 3). Improved sludge settling, dewatering characteristics, bacterial inactivation capability, sludge stability are reported to be the major advantages of lime-based coagulation [34,35].
In spite of its advantages, coagulation–flocculation has limitations such as high operational cost due to chemical consumption. The increased volume of sludge generated from coagulation–flocculation may hinder its adoption as a global strategy for wastewater treatment. This can be attributed to the fact that the toxic sludge must be converted into a stabilized product to prevent heavy metals from leaking into the environment [36].
To overcome such problems, electro-coagulation may be a better alternative than conventional coagulation, as it can remove the smallest colloidal particles and produce just a small amount of sludge [37,38]. However, this technique also creates a floc of metallic hydroxides, which requires further purification [39], making the recovery of valuable heavy metals impossible.

2.3. Flotation
Flotation is employed to separate solids or dispersed liquids from a liquid phase using bubble attachment [40]. The attached particles are separated from the suspension of heavy metal by the bubble rise. Flotation can be classified as: (i) dispersed-air flotation, (ii) dissolved-air flotation (DAF), (iii) vacuum air flotation, (iv) electro flotation, and (v) biological flotation. Among the various types of flotation, DAF is the most commonly used for the treatment of metal-contaminated wastewater [41]. Adsorptive bubble separation employs foaming to separate the metal impurities. The target floating substances are separated from bulk water in a foaming phase.
Laboratory study was carried out by Rubio and Tessele [42] to investigate the flotation of Zn(II) and Ni(II) from synthetic wastewater using chabazite as the adsorptive particulate. They found that the removal performance was dependent on the interfacial chemistry and aggregation effectiveness. An almost complete removal (98.6%) of heavy metal ions with an initial concentration of 2 mg/L could be achieved by using 20 mg/L of Fe(OH)3. The results are comparable to those of Blocher et ¨ al. [43], who combined flotation and membrane separations to remove Ni(II) cations from synthetic plating solution by using CTABr (cetyl trimethyl-ammonium bromide) as the cationic collector (Table 4).
Other interesting results are reported by Zamboulis et al. [44], who studied the sorptive flotation for the removal of Zn(II) and Cu(II) ions from synthetic wastewater. SDS (sodium dodecyl sulfate) and HDTMA (hexadecyl-trimethyl-ammoniumbromide) were used as the cationic collectors. The addition of 2 g/L of zeolite was found to remove 99% of 50 mg/L of zinc(II), while 4 g/L of zeolite was required to remove 97% of 500 mg/L of the initial Cu(II) concentration (Table 4). The results confirmed that both the surface charge of the system and the solution pH significantly affected the metal removal by zeolite [10].
To explore their application as biosurfactants, Surfactin105 and Lychenysin-A were applied to enhance the removal of Cr(VI) and Zn(II) ions from synthetic wastewater [45]. An almost complete removal of both metals with initial metal concentrations of 50 mg/L could be achieved at pH 4.0.
In the last decade, the trends of research had shifted from flotation alone to a combination of flotation and other physico–chemical treatments such as filtration or powder activated carbon [46,47]. Encouraging results were reported by Mavrov et al. [48], who examined a newly integrated process that combined adsorption, membrane separation and flotation for Cu(II) and Zn(II) removal from real wastewater with synthetic zeolite as the bonding agent. They found that about 97% of Cu(II) and Zn(II) removal were attained with an initial metal concentration of 60 mg/L. The binding capacities of Zn(II), Cu(II) and Ni(II) ions were found to be 270, 200 and 60 mg/g, respectively. The results were higher than those of Doyle and Liu [11], who employed triethylenetetraamine (Trien) as the collector for the flotation of Cu(II) ions from synthetic wastewater (Table 4).
Although it is only a kind of physical separation process, heavy metal removal by flotation has the potential for industrial application [49]. Low-cost materials such as zeolite and chabazite have been found to be effective collectors with removal efficiency of higher than 95% for an initial metal concentration ranging from 60 to 500 mg/L (Table 4). Flotation can be employed to treat inorganic effluent with a metal concentration of less than 50 mg/L or higher than 150 mg/L. Other advantages such as better removal of small particles, shorter hydraulic retention times and low cost make flotation one of the most promising alternatives for the treatment of metal-contaminated wastewater [50,51].
2.4. Membrane filtration
Membrane filtration has received considerable attention for the treatment of inorganic effluent, since it is capable of removing not only suspended solid and organic compounds, but also inorganic contaminants such as heavy metals. Depending on the size of the particle that can be retained, various types of membrane filtration such as ultrafiltration, nanofiltration and reverse osmosis can be employed for heavy metal removal and are presented as follows.
2.4.1. Ultrafiltration (UF)
UF utilizes permeable membrane to separate heavy metals, macromolecules and suspended solids from inorganic solution on the basis of the pore size (5–20 nm) and molecular weight of the separating compounds (1000–100,000 Da) [52]. These unique specialties enable UF to allow the passage of water and low-molecular weight solutes, while retaining the macromolecules, which have a size larger than the pore size of the membrane [53].
Some significant findings were reported by Juang and Shiau [14], who studied the removal of Cu(II) and Zn(II) ions from synthetic wastewater using chitosan-enhanced membrane filtration. The amicon-generated cellulose YM10 was used as the ultrafilter. About 100% and 95% rejection were achieved at pH ranging from 8.5 to 9.5 for Cu(II) and Zn(II) ions, respectively, with an initial Cu(II) concentration of 79 mg/L and Zn(II) concentration of 81 mg/L. The results indicated that chitosan significantly enhanced metals removal by 6–10 times compared to using membrane alone. This could be attributed to the major role of the amino groups of the chitosan chain, which served as coordination sites for metal binding. In acidic conditions, the amino groups of chitosan are protonated after reacting with H+ ions as follows:
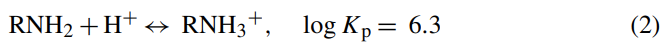
Having the unshared electron pair of the nitrogen atom as the sole electron donor, the non-protonated chitosan binds with the unsaturated transition metal cation through the formation of coordination bond [54]. For most of the chelating adsorbent, the functional groups with the donor atoms are normally attached to the metal ions, thus leading to a donor–acceptor interaction between chitosan and the metal ions [55], as indicated by the
Eq. (3):
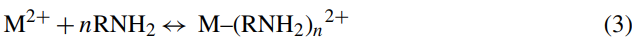
where M and RNH2 represent metal and the amino group of chitosan, respectively, while n is the number of the unprotonated chitosan bound to the metal. Combination of Eqs. (2) and (3) gives the overall reaction as follows:
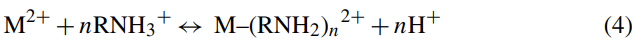
Eq. (4) suggests that an increase in pH would enhance the formation of metal–chitosan complexes.
To improve the rejection rates of metals by complexation– ultrafiltration, some modifications were conducted using polyethylene-imine (PEI), a water-soluble macroligand, to remove Cr(III) ions from a synthetic solution [56]. Technical parameters such as pH, ligand concentration, applied pressure and membrane pore size were found to significantly affect the rejection rate of metal ions. The researchers found that pH 6.0, pressure of 3 bar and 2 g/L of PEI were the optimum conditions to achieve the Cr rejection rate of 95% with an initial metal concentration of 20 mg/L (Table 5).
Another significant breakthrough in UF research was explored for the removal of Ni(II) ions from a synthetic solution using micellar-enhanced UF [57]. Both sodium dodecyl sulfate (SDS) and non-ionic mono-alkylphenol polyetoxilate were used to form micelles. An almost complete removal of Ni(II) could be achieved at 4 bar of pressure with 1 g/L of SDS concentration. This result was higher than that of Akita et al. [58], who studied the removal of Ni(II) ions from synthetic wastewater via micellar-enhanced UF. They found that only 60% of Ni(II) with an initial metal concentration of 29 mg/L was removed at pH ranging from 5 to 7, confirming that the metal rejection rates were dependent on the degree of complexation between the cations and the extractant within the micelle [59].
To explore its potential to remove heavy metals, Saffaj et al. [60] employed low cost ZnAl2O4–TiO2 UF membranes for the removal of Cd(II) and Cr(III) ions from synthetic solution. They reported that 93% Cd(II) rejection and 86% Cr(III) rejection were achieved (Table 5). Such high rejection rates might be attributed to the strong interactions between the divalent cations and the positive charge of the membranes. These results indicate that the charge capacity of the UF membrane, the charge valencies of the ions and the ion concentration in the effluent, played major roles in determining the ion rejection rates by the UF membranes [61].
Depending on the membrane characteristics, UF can achieve more than 90% of removal efficiency with a metal concentration ranging from 10 to 112 mg/L at pH ranging from 5 to 9.5 and at 2–5 bar of pressure (Table 5). UF presents some advantages such as a lower driving force and a smaller space requirement due to its high packing density. However, the decrease in UF performance due to membrane fouling has hindered it from a wider application in wastewater treatment. Fouling has many adverse effects on the membrane system such as flux decline, an increase in transmembrane pressure (TMP) and the biodegradation of the membrane materials [62]. These effects result in high operational costs for the membrane system.
References
[1] S. Babel, T.A. Kurniawan, Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan, Chemosphere 54 (7) (2004) 951–967.
[2] T.A. Kurniawan, A research study on Cr(VI) removal from electroplating wastewater using chemically modified low-cost adsorbents and commercial activated carbon, Sirindhorn International Institute of Technology (SIIT), Thammasat University, Pathumthani, ISBN 974- 570-828-3 (Master thesis), 2002.
[3] US Environmental Protection Agency (EPA), Development Document for Effluent Limitations Guidelines and Standards for the Metal Finishing Point Source Category, US EPA, Washington, DC, 1980 (EPA440/1-80/091-a).
[4] US Environmental Protection Agency (EPA), Control and treatment technology for the metal finishing industry, sulfide precipitation, Summary Report, US EPA, Washington, DC, (EPA-625/8-80/003), 1980.
[5] Environmental Protection Department (EPD), Hong Kong, Technical memorandum standards for effluents discharged into drainage and sewerage systems, inland and coastal water, 2005 [].
[6] Pollution Control Department (PCD), Thai Ministry of Natural Resources and Environment, Water quality standards, 2005 [].
[7] L. Charerntanyarak, Heavy metals removal by chemical coagulation and precipitation, Water Sci. Technol. 39 (10/11) (1999) 135–138.
[8] O. Tunay, N.I. Kabdasli, Hydroxide precipitation of complexed metals, ¨ Water Res. 28 (10) (1994) 2117–2124.
[9] Y.J. Li, X.P. Zeng, Y.F. Liu, S.S. Yan, Z.H. Hu, Ya-Ming, Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation, Sep. Purif. Technol. 31 (2003) 91–95.
[10] N.K. Lazaridis, K.A. Matis, M. Webb, Flotation of metal-loaded clay anion exchangers. Part I: the case of chromate, Chemosphere 42 (2001) 373–378.
[11] F.M. Doyle, Z.D. Liu, The effect of triethylenetetraamine (trien) on the ion flotation of Cu2+ and Ni2+, J. Coll. Int. Sci. 258 (2003) 396–403.
[12] M. Pansini, C. Colella, M. De’Gennaro, Chromium removal from water by ion exchange using zeolite, Desalination 83 (1991) 145–157.
[13] E. Alvarez-Ayuso, A. Garc ´ ´谋a-Sanchez, X. Querol, Purification of metal ´ electroplating wastewaters using zeolites, Water Res. 37 (20) (2003) 4855–4862.
[14] R.S. Juang, R.C. Shiau, Metal removal from aqueous solutions using chitosan-enhanced membrane filtration, J. Membr. Sci. 165 (2000) 159–167.
[15] K.H. Ahn, K.G. Song, H.Y. Cha, I.T. Yeom, Removal of ions in nickel electroplating rinse water using low-pressure nanofiltration, Desalination 122 (1999) 77–84.
[16] A.J. Pedersen, Characterization and electrolytic treatment of wood combustion fly ash for the removal of cadmium, Biomass Bioenergy 25 (4) (2003) 447–458.
[17] L.J.J. Janssen, L. Koene, The role of electrochemistry and electrochemical technology in environmental protection, Chem. Eng. J. 85 (2002) 137–146.
[18] N. Kongsricharoern, C. Polprasert, Electrochemical precipitation of chromium (Cr6+) from an electroplating wastewater, Water Sci. Technol. 31 (9) (1995) 109–117.
[19] S. Babel, T.A. Kurniawan, Various treatment technologies to remove arsenic and mercury from contaminated groundwater: an overview, in: Proceedings of the 1st International Symposium on Southeast Asian Water Environment, Bangkok, Thailand, 24–25 October, 2003, pp. 433–440.
[20] L.D. Benefield, J.M. Morgan, Chemical precipitation, in: R.D. Letterman (Ed.), Water Quality and Treatment, McGraw-Hill Inc., NY, 1999, pp. 10.1–10.57.
[21] US Environmental Protection Agency (EPA), Chemical Precipitation, US EPA, Washington, DC, 2000 (EPA832-F-00-018).
[22] L.K. Wang, D.A. Vaccari, Y. Li, N.K. Shammas, Chemical precipitation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 141–198.
[23] O. Tunay, Developments in the application of chemical technologies ¨ to wastewater treatment, Water Sci. Technol. 48 (11/12) (2003) 43–52.
[24] O. Tunay, N.I. Kabdasli, R. Tasli, Pretreatment of complexed metal ¨ wastewaters, Water Sci. Technol. 29 (9) (1994) 265–274.
[25] A. Papadopoulos, D. Fatta, K. Parperis, A. Mentzis, K.J. Harambous, M. Loizidou, Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods, Sep. Purif. Technol. 39 (3) (2004) 181–188.
[26] K. Juttner, U. Galla, H. Schmieder, Electrochemical approaches to ¨ environmental problems in the process industry, Electrochim. Acta 45 (2000) 2575–2594.
[27] X.J. Yang, A.G. Fane, S. MacNaughton, Removal and recovery of heavy metals from wastewater by supported liquid membranes, Water Sci. Technol. 43 (2) (2001) 341–348.
[28] P. Bose, M.A. Bose, S. Kumar, Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc, and cyanide, Adv. Environ. Res. 7 (2002) 179–195.
[29] U. Wingenfelder, C. Hansen, G. Furrer, R. Schulin, Removal of heavy metals from mine water by natural zeolites, Environ. Sci. Technol. 39 (2005) 4606–4613.
[30] N.K. Shammas, Coagulation and flocculation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 103–140.
[31] L. Semerjian, G.M. Ayoub, High-pH-magnesium coagulation–flocculation in wastewater treatment, Adv. Environ. Res. 7 (2003) 389– 403.
[32] I. Licsko, Realistic coagulation mechanisms in the use of aluminium ´ and iron(III) salts, Water Sci. Technol. 36 (4) (1997) 103–110.
[33] M.E. Andrus, A review of metal precipitation chemicals for metalfinishing applications, Met. Finish. 98 (11) (2000) 20–23.
[34] R.C. Cheng, S. Liang, H.C. Wang, M.D. Beuhler, Enhanced coagulation for arsenic removal, J. AWWA 86 (9) (1994) 79–90.
[35] M. Edwards, Chemistry of arsenic removal during coagulation and Fe–Mn oxidation, J. AWWA 86 (9) (1994) 64–78.
[36] G.M. Ayoub, L. Semerjian, A. Acra, M. El Fadel, B. Koopman, Heavy metal removal by coagulation with seawater liquid bittern, J. Environ. Eng. 127 (3) (2001) 196–202.
[37] E.I. Vik, D.A. Carlsoon, A.S. Eikum, E.T. Gjessing, Electrocoagulation of potable water, Water Res. 18 (1984) 1355–1360.
[38] M. Elimelech, C.R. O’Melia, Kinetics of deposition of colloidal particles in porous media, Environ. Sci. Technol. 24 (1990) 1528–1536.
[39] F. Persin, M. Rumeau, Le traitement electrochimique des eaux et des ´ effluents, Tribune de l’eau 3 (42) (1989) 45–46. [40] L.K. Wang, E.M. Fahey, Z.C. Wu, Dissolved air flotation, in: L.K. Wang, Y.T. Hung, N.K. Shammas (Eds.), Physicochemical Treatment Processes, vol. 3, Humana Press, New Jersey, 2004, pp. 431–500.
[41] T. Zabel, Flotation in water treatment, in: K.J. Ives (Ed.), The Scientific Basis of Flotation, Martinus Nijhoff Publishers, The Hague, 1984, pp. 349–378.
[42] J. Rubio, F. Tessele, Removal of heavy metal ions by adsorptive particulate flotation, Min. Eng. 10 (7) (1997) 671–679.
[43] C. Blocher, J. Dorda, V. Mavrov, H. Chmiel, N.K. Lazaridis, K.A. ¨ Matis, Hybrid flotation-membrane filtration processes for the removal of heavy metal ions from wastewater, Water Res. 37 (2003) 4018–4026.
[44] D. Zamboulis, S.I. Pataroudi, A.I. Zouboulis, K.A. Matis, The application of sorptive flotation for the removal of metal ions, Desalination 162 (2004) 159–168.
[45] A.I. Zouboulis, K.A. Matis, N.K. Lazaridis, P.N. Golyshin, The use of biosurfactants in flotation: application for the removal of metal ions, Min. Eng. 16 (2003) 1231–1236.
[46] G. Offringa, Dissolved air flotation in Southern Africa, Water Sci. Technol. 31 (3/4) (1995) 159–172.
[47] S. Laine, T. Poujol, S. Dufay, J. Baron, P. Robert, Treatment of ´ stormwater to bathing water quality by dissolved air flotation, filtration and ultraviolet disinfection, Water Sci. Technol. 38 (10) (1998) 99–105.
[48] V. Mavrov, T. Erwe, C. Blocher, H. Chmiel, Study of new integrated ¨ processes combining adsorption, membrane separation and flotation for heavy metal removal from wastewater, Desalination 157 (2003) 97–104.
[49] P. Jokela, P. Keskitalo, Plywood mill water system closure by dissolved air flotation treatment, Water Sci. Technol. 40 (11/12) (1999) 33–42.
[50] K.A. Matis, A.I. Zouboulis, N.K. Lazaridis, I.C. Hancock, Sorptive flotation for metal ions recovery, Int. J. Miner. Process 70 (2003) 99–108.
Jul 12,2024
